thermochemical sulfate reduction (tsr) is a common organic-inorganic interaction in sedimentary basins. tsr is in close association with hydrocarbon evolution and metal sulfide precipitation in the mississippi valley-type deposits. currently, the reaction pathway and mechanism of tsr remain controversial, hindering our understanding of metal sulfide precipitation and hydrocarbon evolution. sulfur species involved in tsr reaction are highly complex, with some intermediate sulfur species being stable only at elevated temperatures (e.g., s3-, stable at >150℃). additionally, sulfur species can react with the metallic chamber at elevated t-p conditions, making traditional autoclave quenching method ineffective for investigating sulfur transformation pathway during tsr. in contrast, fused silica capillary capsules (fsccs) serve as ideal micro-reactors for simulating tsr experiments because they are inert to sulfur species and allow online optical and spectroscopic observations. utilizing fsccs and in situ raman spectroscopic observation techniques, professor xiaolin wang's group successfully characterized sulfur transformation pathway and conducted systematic quantification analyses and simulations. these works aim to reveal the mechanism and kinetics of the tsr reaction.
their results indicate that sulfate is transformed to h2s through a series of reduction and disproportionation reactions. for example, in the na2so4-h2so4-ch4-n2-h2o system (fig. 1, where n2 is used as an internal reference), sulfate is first reduced by methane to form so2. then, so2 is further disproportionated or reduced to form h2s. the interaction between high valence sulfur species and h2s leads to the formation of intermediate valence sulfur species (i.e., s3- and s0), which directly oxidize methane to form h2s and co2. this study provides direct evidence that both s3- and s0 can oxidize hydrocarbon, contributing to the autocatalytic effect observed in the tsr reaction. the transformation of sulfur species during tsr reaction is illustrated in fig. 2.
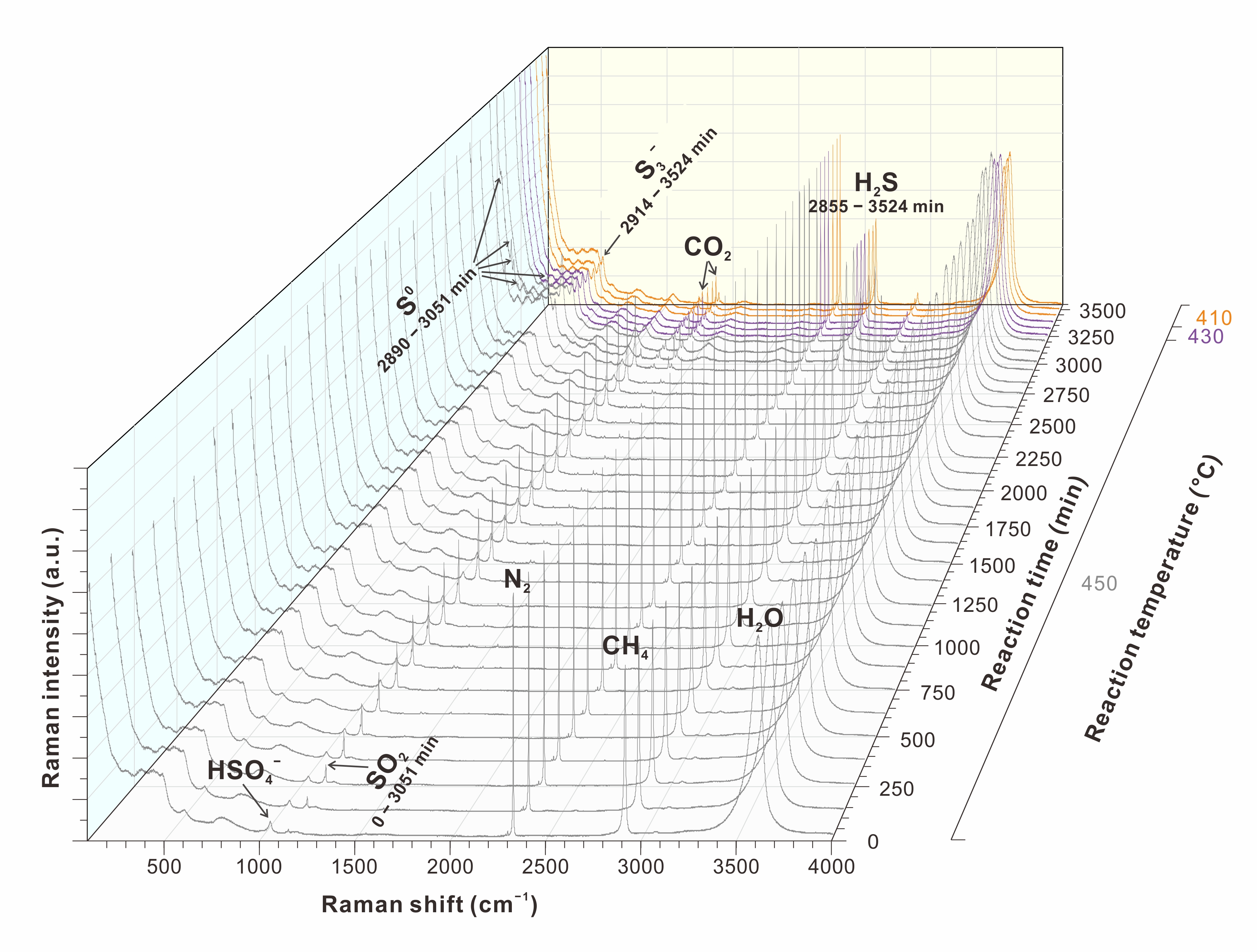
fig. 1 variation of the in situ raman spectra for the sulfate-poor liquid phase of the na2so4-h2so4-ch4 system as a function of temperature (°c) and reaction time (min). all spectra are normalized with respect to the intensity of the ν1(n2) band.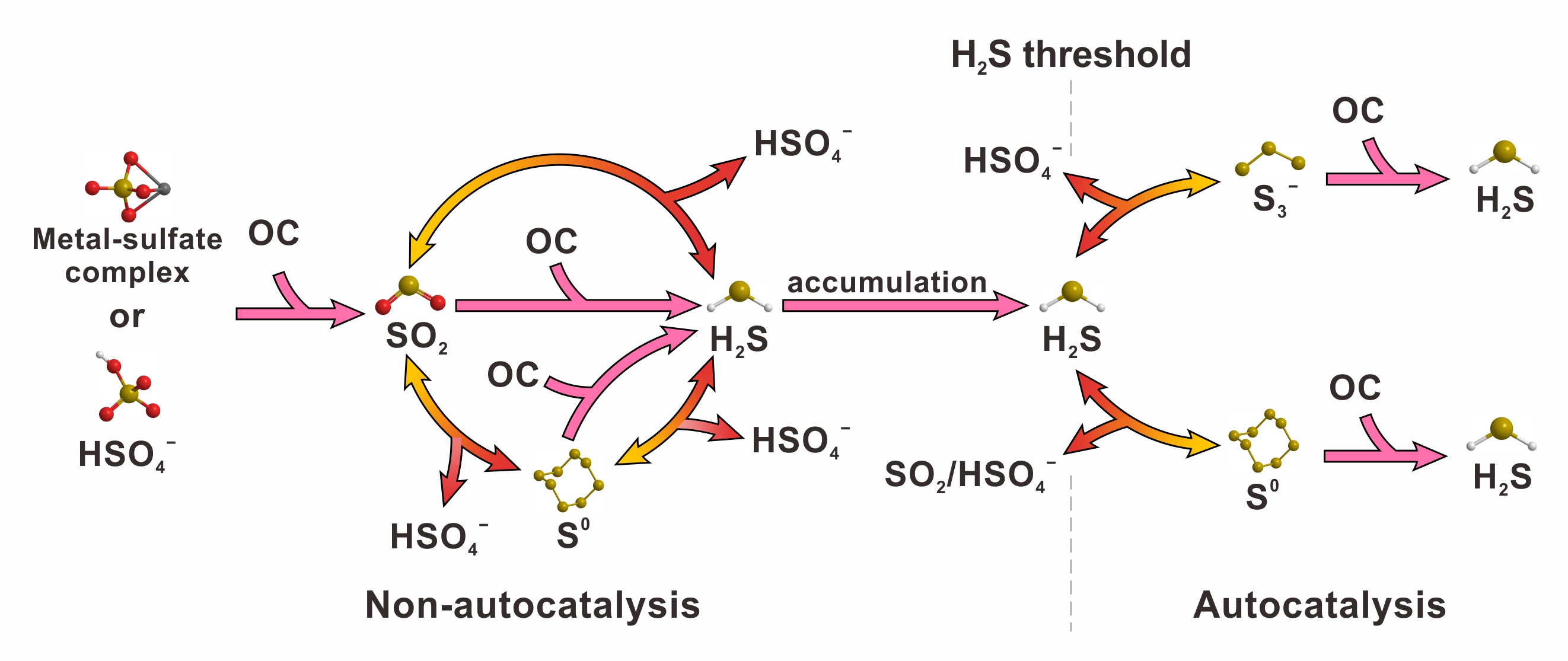
fig. 2 the transformation of sulfur species during tsr reaction. oc denotes organic compound. double-headed arrow represents a reversible reaction.
the transformation of sulfur species significantly affects the kinetics of tsr. based on this transformation, tsr reaction can be divided into three stages (fig. 3). in the low-rate sulfate reduction (lrsr) stage, hydrocarbon is oxidized by sulfate at a low rate. in the subsequent high-rate s0 (and so2) reduction (hrsr) stage, hydrocarbon is mainly oxidized by s0 (and so2). the last stage is the medium-rate s3- reduction (mrsr) stage, where s3- played a crucial role in hydrocarbon oxidation. this study represents the first comprehensive characterization of the kinetics of these stages separately. utilizing the obtained kinetic parameters, we simulated tsr-induced mineralization of mvt deposits (fig. 4). the simulation results indicate that in situ tsr can provide sufficient reduced sulfur for the precipitation of mental sulfide, demonstrating that in situ tsr is an efficient mineralizing mechanism for mvt deposits. based on the extrapolated kinetic parameters, the half-life of methane in methane-dominated tsr reaction at 200 °c is only 7.1 myr. this finding suggests that even the most stable hydrocarbon, methane, can be quickly consumed by tsr in reservoirs. consequently, tsr is an important mechanism resulting in the chemical consumption of hydrocarbons in petroliferous basins.
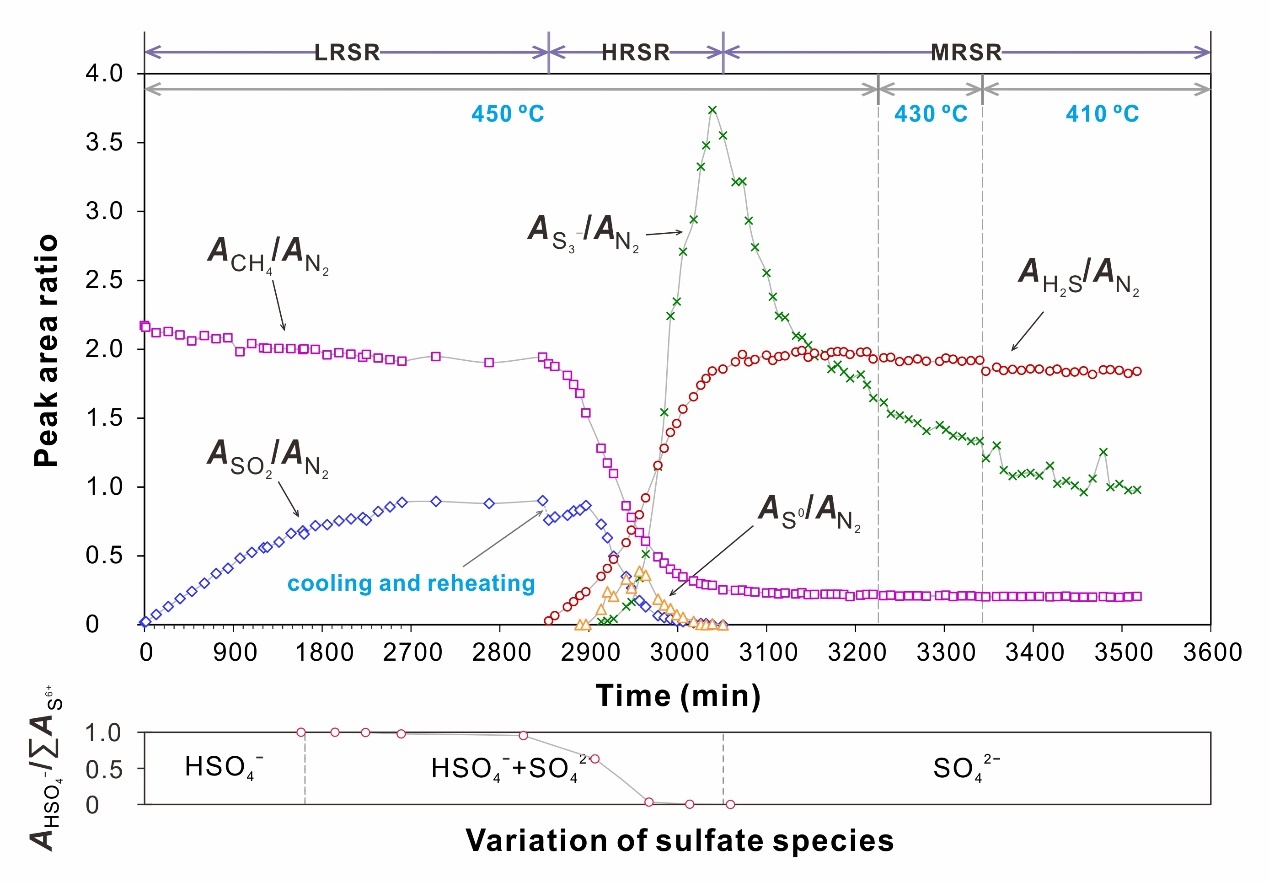
fig. 3 variation of the peak area ratios between the tsr-involved species and n2 with experimental time, and the division of the reaction stages for the na2so4-h2so4-ch4 system.
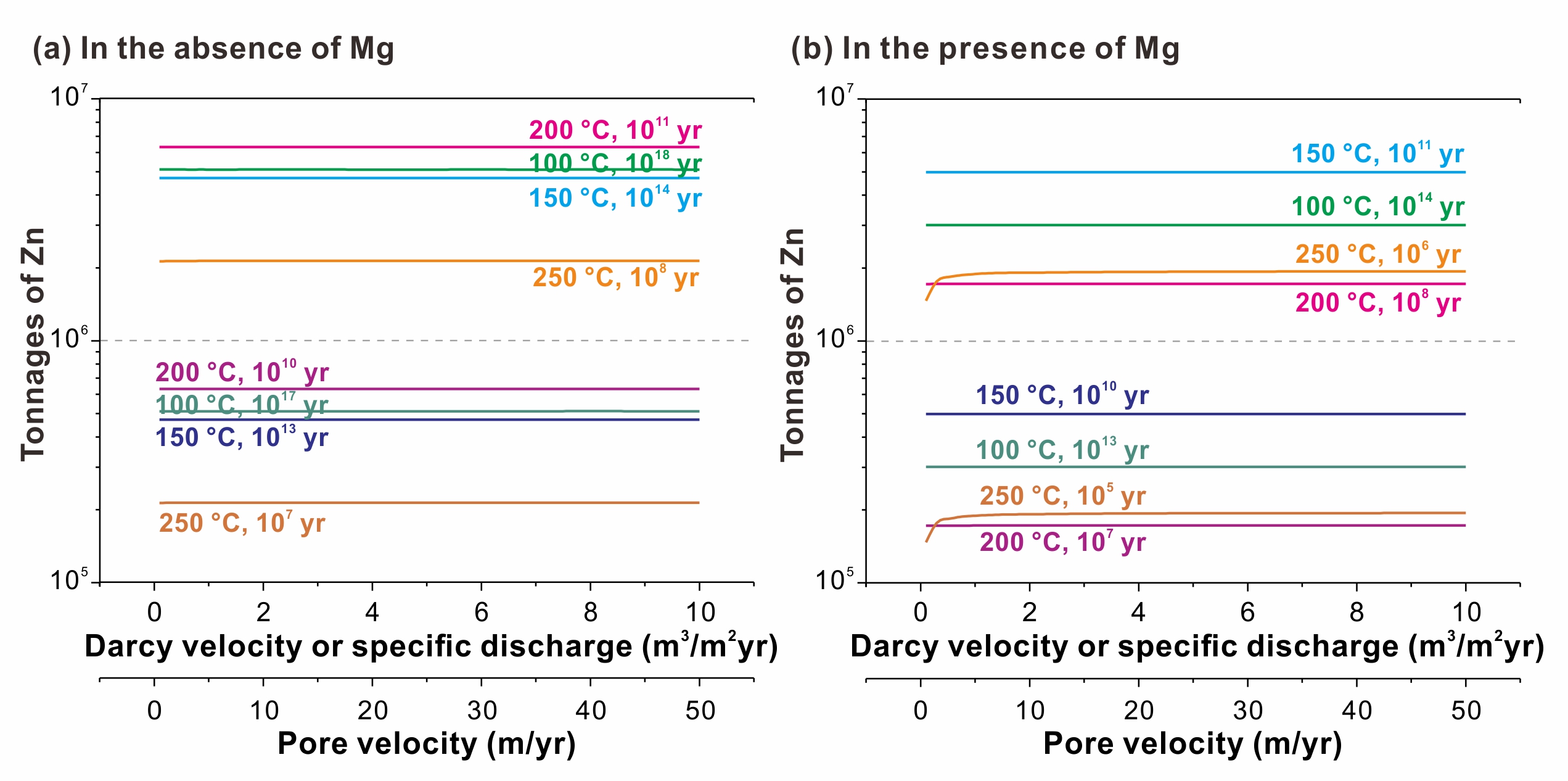
fig. 4 tonnages of zn precipitation from mvt deposits induced by in situ tsr.
this contribution, entitled by “reaction pathway, mechanism and kinetics of thermochemical sulfate reduction: insights from in situ raman spectroscopic observations at elevated temperatures and pressures” was published in geochimica et cosmochimica acta (381: 25-42; https://www.sciencedirect.com/science/article/pii/s0016703724003752). the first author is mr qian wan (phd candidate), and the corresponding author is professor xiaolin wang. the coauthors include professor wenxuan hu, associate research fellow ye wan and research fellow i-ming chou from the institute of deep-sea science and engineering, chinese academy of sciences. this research is mainly supported by the national key research and development program of china (2022yff0800403), the national natural science foundation of china (42173038), the research funds for the frontiers science center for critical earth material cycling (nanjing university).

